Lymphocyte population characterization in ovarian cancer microenvironment
DOI:
https://doi.org/10.48797/sl.2025.306Keywords:
PosterAbstract
Background: Ovarian cancer (OC) is one of the most common gynecological cancers, with poor prognosis and high mortality [1]. A key early sign of OC is the presence of ascites [2], indicating transcoelomic metastasis and tumor spread within the peritoneal cavity [3]. The accumulation of this serous fluid facilitates migration of tumor cells, which detach from the primary tumor (PT) in multicellular aggregates, migrate through ascitic fluid, and establish metastatic implants (MI) [3]. Ascites also contains acellular components and other cells, such as immune cells. In fact, the interactions between all these components play a crucial role in disease progression [2,3]. Since transcoelomic metastasis involves changes in tumor cell differentiation, characterizing and comparing the tumor immune microenvironment (TIME) in both PT and MI is crucial to assess whether therapies targeting PT are also effective against MI. Objective: We aimed to a better understanding of the TIME in OC patients, evaluating and comparing the populations of T lymphocytes, B lymphocytes and macrophages in PT and their respective MI. Methods: We used a tissue microarray with samples of PT and MI from each patient, which included 13 cases with histological diagnosis of high-grade serous carcinoma that have not undergone chemotherapy treatment to: conduct a qualitative assessment of matrix elements using histochemical techniques (trichrome staining for collagen, orcein staining for elastin and silver staining for reticulin); perform a semi-quantitative assessment of different leucocyte populations through IHC staining with anti-CD3 (T lymphocytes), anti-CD20 (B lymphocytes) and anti-CD163 (macrophages); evaluate and quantify the immune cells using two different methods (manual semi-quantitative evaluation under LM and automatic quantitative evaluation using QuPath, Figure 1). Results: Collagen fibers were observed in the stroma of both PT and MI, whereas no elastic fibers were observed; reticular fibers were present in rich lymphoid tissue areas. The presence of T and B lymphocytes, as well as macrophages, was higher in MI than in PT. There was a greater abundance of T lymphocytes than B lymphocytes in the stroma. Finally, the macrophage population was more abundant than the lymphocyte population, both in PT and MI. Conclusions: The differences observed between PT and MI show an immune system adaptation that can influence the progression of the disease, its prognosis, and treatment response.
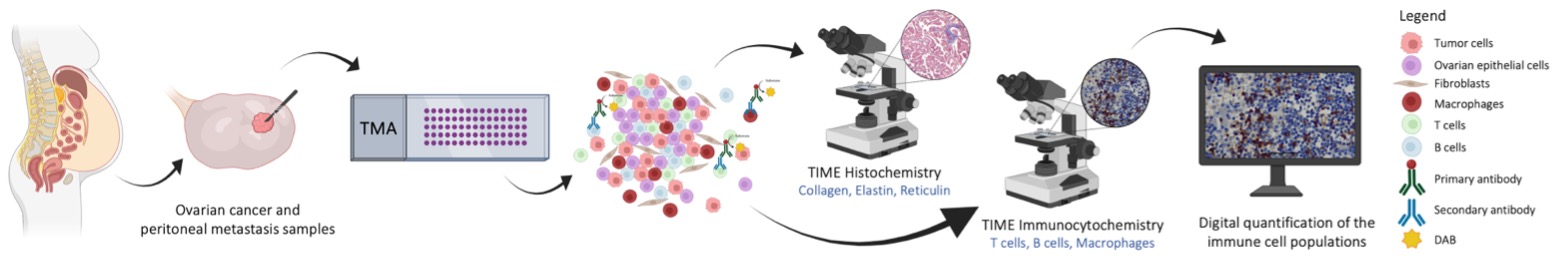
Figure 1. Overview of the experimental approach used to evaluate the populations of T lymphocytes, B lymphocytes and macrophages in PT and MI.
References
1. James, F. R. et al. Association between tumour infiltrating lymphocytes, histotype and clinical outcome in epithelial ovarian cancer. BMC Cancer 2017, 17(1), 657, doi: 10.1186/s12885-017-3585-x
2. Baci, D. et al. The Ovarian Cancer Tumor Immune Microenvironment (TIME) as Target for Therapy: A Focus on Innate Immunity Cells as Therapeutic Effectors. International Journal of Molecular Sciences 2020, 21(9), 3125, doi : 10.3390/ijms21093125
3. Nunes, D. et al. Ovarian Cancer Ascites as a Liquid Tumor Microenvironment. Exon Publications 2022, 43–55, doi: 10.36255/exon-publications-ovarian-cancer-tumor-microenvironment
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Ana Rita Nunes, Tomás Rodrigues, Fernanda Garcez, Sara Ricardo, Albina Dolores Resende, Carla Batista-Pinto

This work is licensed under a Creative Commons Attribution 4.0 International License.
In Scientific Letters, articles are published under a CC-BY license (Creative Commons Attribution 4.0 International License), the most open license available. The users can share (copy and redistribute the material in any medium or format) and adapt (remix, transform, and build upon the material for any purpose, even commercially), as long as they give appropriate credit, provide a link to the license, and indicate if changes were made (read the full text of the license terms and conditions of use).
The author is the owner of the copyright.









